In breakthrough, Israeli scientists say they synthesized human embryos from stem cells
Weizmann Institute team uses stem cell model allowing researchers to see into ‘black box’ of critical early stage of pregnancy
Renee Ghert-Zand is a reporter and feature writer for The Times of Israel.
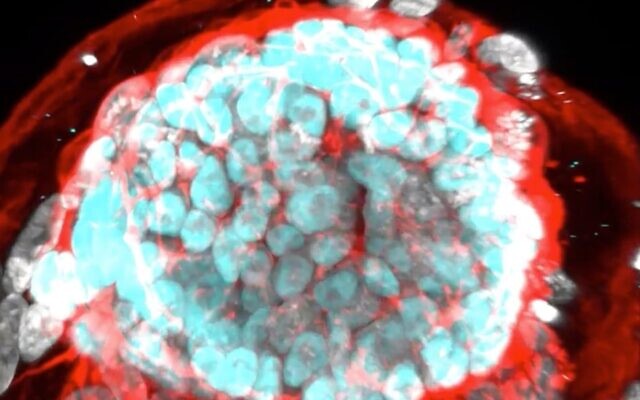
After a decade of work, researchers at the Weizmann Institute of Science say they have managed to create stem cell-derived human embryo models outside the uterus.
The advance could help scientists better understand healthy and unhealthy embryonic development. It also has implications for studying the genetics of various organs, the possible growing of organs for transplantation, and the investigation of the effects of pharmaceuticals on embryos.
The discovery was shared Thursday in a pre-print version of a paper that is in advanced stages of revision at a prestigious peer-reviewed journal.
The stem cell-derived embryo (SEM), which undergoes no genetic manipulation, is equivalent to a 14-day-old embryo. It has an embryo proper, a yolk sac, an amniotic cavity, a placenta and cells of the chorion, or the outermost fetal membrane.
“It does not have a beating heart or a brain but it has a very complex organization and is already starting to show early differentiation of tissues. All the elements of the architecture are there and they are in the proper relation and orientation to each other,” said Prof. Jacob Hanna.
Hanna and his team at Weizmann’s department of molecular genetics were the first to publish reports of success in creating mouse SEMs in Cell in 2022 and Nature in 2021.
Now they’ve made human SEMs through a complicated process informed in part by the study of the animal models. There was much experimentation until they found the right protocol.
“We had to create induced pluripotent stem cells and nudge them to become the yolk sac, the chorion, trophoblasts and so on. And what was the ratio and how should we combine them. Then we put them on a shaker and changed the media protocol,” Hanna told The Times of Israel on Thursday.
“Some of the media we used had been discussed before, but the critical three media are newly used by our group and described in our paper,” he added.
This breakthrough comes on the heels of an article in The Guardian about an announcement Wednesday at the International Society for Stem Cell Research annual meeting in Boston of the creation of a synthetic human embryo by Prof. Magdalena Żernicka-Goetz, of the University of Cambridge and the California Institute of Technology.

Hanna, who has shared his latest discovery internally within the medical community for the past six months, dismissed Żernicka-Goetz’s announcement as premature.
“Her claims are outrageous. She doesn’t even have a pre-print article published. I watched her presentation and saw the few slides she showed. Her model doesn’t have a placenta or a yolk sac, so it cannot be called an embryo. I wouldn’t call it a success,” he said.
Although it has been possible since 2006 to revert blood or skin cells from human adults to embryonic stem cells in the petri dish, differentiation still proves difficult.
“We generally cannot mimic the very complex processes that happen during embryo development. To do this, we need to learn what are the genes and the proteins that are turned on or off during this development process. Once we uncover them, we can add them or reduce them. So that is why we need to mimic the embryo,” Hanna said.
“But we don’t know the human embryo. It makes all of its organs between day seven and day 28 of pregnancy. After that, it’s just about growth for the next eight months. So you have this rapid, critical three weeks in the beginning when you have all this drama happening, but it’s a black box,” he explained.
There are no embryos at that stage for researchers to study because most women don’t even know they are pregnant during those first weeks. If they have a miscarriage or spontaneous abortion, they wouldn’t even know.
However, should a woman know she is pregnant and choose an abortion at that stage, it would generally be done medically. A surgical abortion, which is unlikely, would damage the embryo. Neither of these options would supply researchers with embryos to study, and certainly not in the large quantities needed.
“So what we have is a way to see inside that proverbial black box. With our SEM we have a model system [extremely close to what you would find in a pregnant woman’s uterus] that allows us to study problems with the development of the yolk sack, for instance. Or we can learn about why the neural tube does not close properly during that stage,” Hanna said.
Hanna was quick to point out and was adamant that creating two-to-three millimeter-long SEMs is not about growing them to term in-utero or in-vitro. Human pregnancy is just too long and complicated for the latter. The former simply doesn’t work biologically.
“Aside from the fact that it is illegal, it is impossible to do. Even if somebody wanted to, you can put it back into the uterus only until the 64-cell stage of the blastocyst. Half a day later in any species, you put it back into the uterus and it does not implant. This is true even for natural embryos,” Hanna said.
In the article in The Guardian, Robin Lovell-Badge, the head of stem cell biology and developmental genetics at the Francis Crick Institute in London, said it was impossible to say at this point whether science could overcome this obstacle, thus creating the potential for the SEM to develop into a living being.
“That’s very difficult to answer. It’s going to be hard to tell whether there’s an intrinsic problem with [the SEMs] or whether it’s just technical,” he said.
Should the answer become clearer, bioethicists — both secular and religious — would certainly weigh in, if they do not plan to already.









